FricTest
FricTest
+ Shipping
Couldn't load pickup availability
Description
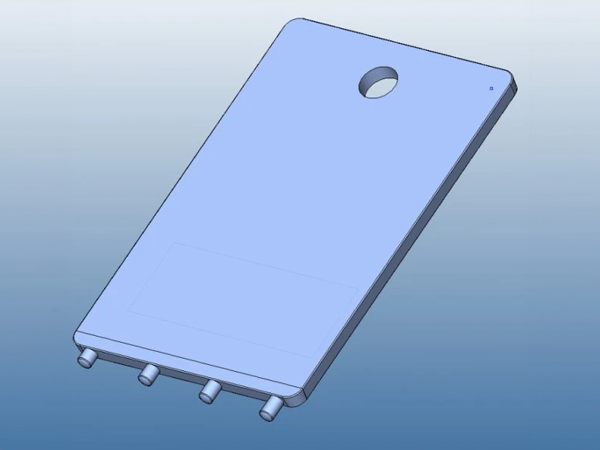
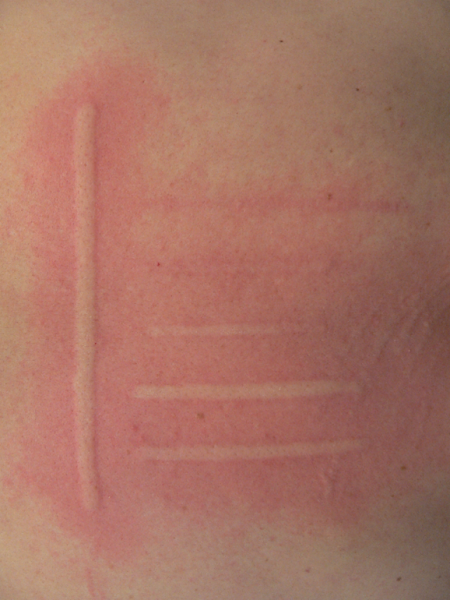
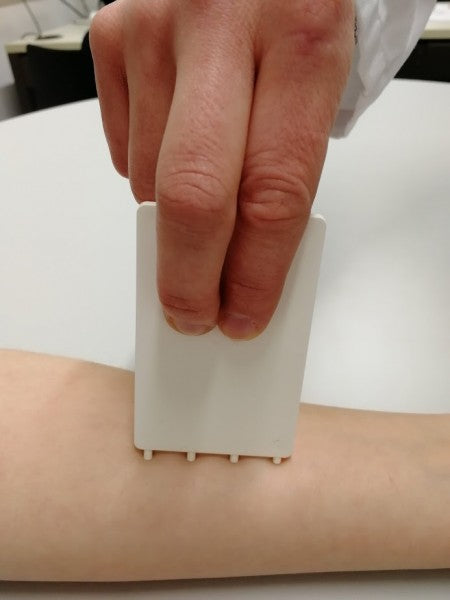
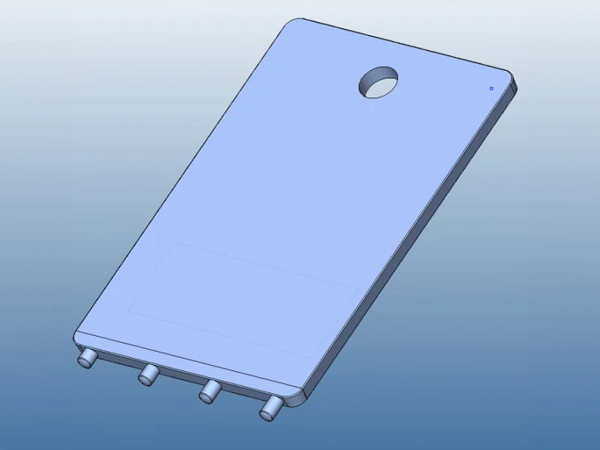
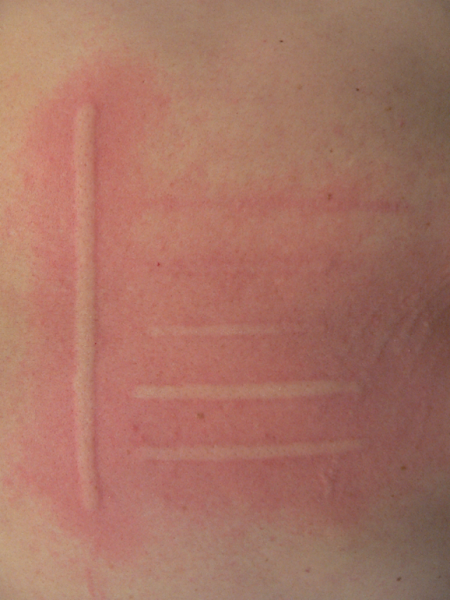
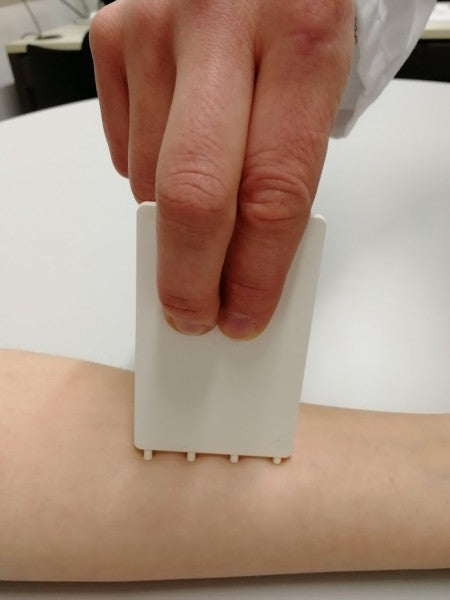
FricTest® is a validated, easy to use dermographometer for diagnosing patients with symptomatic dermographism (urticaria factitia) and for measuring their trigger thresholds and disease activity. The comb-like, four-pronged design of FricTest® 4.0, the newest version of this instrument, allows for simultaneous dermal stimulation with four tips of different lengths exerting four different provocation levels, from I (the weakest) to IV (the strongest). To obtain a response, FricTest® is placed vertically so that the four tips are touching the skin, and then stroked once with moderate pressure across the width of the volar surface of the forearm (or the patient´s back) for a distance of approximately 6 centimeters. A positive response is defined by a palpable weal of ≥3 mm in width (the diameter of FricTest® tips) at 10 min after provocation. Positive test responses to provocation with the longest FricTest® tip provocation level IV) confirm the presence of symptomatic dermographism.
FricTest® was developed by a Charité team of scientists led by Dr. Markus Magerl and Dr. Marcus Maurer. In cooperation with Charité biomedical workshop engineers, the team had first developed several prototypes that were then tested against traditional spring-loaded one-pronged dermographometers in clinical trials. Based on the results of these studies, FricTest® 4.0 was developed together with MOXIE scientists. FricTest® 4.0 was awarded medical product status.
Quick Facts FricTest®
Purpose: Confirm diagnosis of symptomatic dermographism and determine trigger thresholds (disease activity)
Suited for: Use by health care professionals or patients
Test sites: Volar forearm or back are recommended
Test reading: 10 minutes after provocation
Test result: Positive if wheal of ≥3 mm in width
Reference:
Młynek, A.*, Vieira dos Santos, R.*, Ardelean, E., Weller, K., Magerl, M., Church, M. K., and Maurer, M.: A novel, simple, validated and reproducible instrument for assessing provocation threshold levels in patients with symptomatic dermographism. Clin. Exp. Dermatol. 2013: 38; 360-366.
*Both authors contributed equally
Commercial or non-commercial
If you are interested or on request, we will be happy to send you our PROMs (Patient Reported Outcomes Measures) on the various topics by e-mail.
Please understand that we have to charge a fee of 15 Euro per PRO when using our PROMs for commercial purposes. Commercial use include, for example, use in industry-sponsored studies, market research and similar purposes.
By non-commercial use we mean e.g. use for consultation hours in hospitals and private practices, use within scientific studies without sponsoring. The prerequisite is that no commercial interest is pursued.
Frequently Asked Questions
Why are there PRO (Patient Reported Outcomes) products that cost nothing and others that cost 15 Euros?
If you plan to use PRO (Patient Reported Outcomes) products (e.g. AAS, AE-QoL, UCT) commercially, there is a small fee per patient (15 €). This applies, for example, to industry-sponsored trials and clinical studies or market research.
You can order free versions, if you plan to use them for non commercial purposes, e.g. for patient management or for academia-driven non commercial
research projects. When you order PRO (Patient Reported Outcomes) products, these instruments are sent per e-mail.
I would like to use PRO (Patient Reported Outcomes) products commercially. Do I have to buy one version each for every time I plan to use it, even in the same patient?
No. Each PRO (Patient Reported Outcomes) products you purchase can be used multiple time by the same patient.
What do I do if PRO (Patient Reported Outcomes) products are not available for my country or in the language I need?
The version you are looking for is probably already under development. Feel free to get in touch with us by e-mail to check when it will be available.